Enzyme Inhibition
We perform inhibition studies to determine IC50, Ki, and TDI IC50 shift for investigational drugs and ADC metabolites.
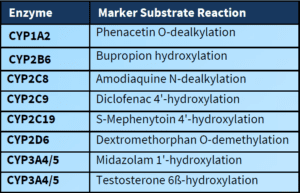
• CYP1A2, CYP2B6, CYP2C8, CYP2C9,CYP2C19, CYP2D6 and CYP3A4/5 included in standard panel using pooled human liver microsomes.
• UGT inhibition also available, which is advised if the drug is metabolized by UGTs.
Enzyme Induction
In vitro CYP induction studies in human hepatocytes are performed using 3 separate donors to meet regulatory guidance.
• CYP1A2, CYP2B6, and CYP3A4 are included in standard panel (mRNA endpoint by RT-qPCR).
• CYP2C8, CYP2C9, and CYP2C19 can and should be evaluated if CYP3A4 induction is observed (marker substrate activity endpoint).
• Cell viability during incubation period is monitored by lactate dehydrogenase activity (LDH).

Drug Transporters
In vitro studies in appropriate cell lines or hepatocytes are designed to assess whether
investigational drugs are transporter substates or inhibitors.
• Efflux transporters: P-gp, BCRP
• Hepatic uptake transporters: OATP1B1, OATP1B3
• Renal transporters: OAT1, OAT3, OCT2, MATE1, and MATE2-K
