Bioconjugation
We have experience producing thousands of batches of ADCs from 1 mg to multi-gram scale including very hydrophobic payloads and difficult antibodies.
Scope
• Small-scale (1 to 100 mg) conjugation for in vitro studies and in vivo efficacy
• Screening of different antibodies and linker-payloads for conjugation CMC properties to select lead candidates
• Conjugation process development
Conjugation types
• Wild-type Cysteine conjugation up to DAR 8
(hIgG1), DAR 10 (mouse) or DAR 12 (hIgG2)
• Site-specific conjugation
• Various conjugation chemistries
• Protein labeling (biotin, fluorescent probes)

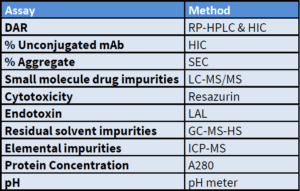
ADC Characterization
Our analytical team can develop and validate
methods for ADCs we produce, or support your
in-house conjugation efforts.
PK/PD Studies
We have validated methods for common payloads, and can quickly develop and validate methods for novel payloads upon request.
• ADC payload and metabolite analysis in plasma and tissues by LC-MS/MS
• mAb and ADC analysis by ELISA

In Vitro Stability
We support optimization of mAb, conjugation site, conjugation handle, linker, or payload with in vitro screening to assess stability in plasma, lysosomes, tissue lysates, or cultured cells. Our capabilities include:
• Enzymatic cleavage kinetic studies for linkers (cathepsins, esterases, ß-glucuronidase, sulfatase, or relevant enzyme/chemical trigger)
• In vitro plasma stability to assess de-conjugation through change in DAR and LC-MS/MS analysis of small molecule release products
